RESEARCH ARTICLE
Effect of resveratrol and its hydroxylated analogues on proliferation and apoptosis of two cervix cancer derived cancer cell lines. The role of mitochondrial superoxide dismutase
Hanna Piotrowska1, Malgorzata Kucinska1, Marcin Wierzchowski2, Urszula Kazmierczak-Majchrzak3, Marek Murias1, *
Article Information
Identifiers and Pagination:
Year: 2013Volume: 4
First Page: 4
Last Page: 13
Publisher Id: TOBCJ-4-4
DOI: 10.2174/1874847320130701002
Article History:
Received Date: 20/5/2013Revision Received Date: 10/6/2013
Acceptance Date: 20/6/2013
epreprint1/7/2013
Electronic publication date: 6/9/2013
Collection year: 2013
open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
The naturally occurring polyphenol resveratrol (3,4',5-trihydroxy-stilbene, 3,4',5-THS, RES) has been shown as a chemopreventive and proapototic agent. Resveratrol is extensively metabolized by CYP450 enzymes. The monohydroxylation of resveratrol is catalyzed by CYP1B1 to form 3,3',4',5-THS (piceatannol), a metabolite with higher anticancer activity and stronger antioxidant properties. It was hypothesized that RES analogues (HHRAs) possessing more than 3 hydroxyl groups may act stronger against cancer cells than RES due to reactive oxygen species formed in redox-cycling reactions. In order to investigate a structure-activity relationship between pro/antioxidant properties and cytotoxicity, the HHRAs with at least 2 phenolic groups in neighborhood- 3,4,4',5-HS and 3,3',4,4',5,5'-HS were synthesized. In the present study we tested this hypothesis in a cell culture model using HeLa and C33A cancer cell lines. The results of our experiments support a hypothesis that MnSOD overexpressing HeLa cells are much more resistant to superoxide generating HHRAs than C33A cells.
INTRODUCTION
Resveratrol (3,4’,5-trihydroxy-trans-stilbene), a phytoalexin showing antioxidant properties, found in grapes and red wine belongs to the most promising natural chemopreventive and anticancer compounds. Several studies indicate that resveratrol can block the process of multistep carcinogenesis, namely, tumor initiation, promotion and- progression [1]. It was shown in our previous research using HL-60 cells that higher hydroxylated resveratrol analogues possessing catechol and/or pyrogallol groups in stilbene rings (HHRAs) are stronger cytotoxic agents than resveratrol. Our experiments showed that such compounds generate ortho-semiquinone intermediates (oSQI) during microsomal oxidation (Fig. 1) [2]. This process undergoes at the expense of oxygen and reducing equivalents, possibly transferred via cytochrome b5 and leads to oSQI redox-cycling resulting in continuous overproduction of superoxide radical. Due to its relatively low reactivity superoxide radical is able to damage only limited number of cellular targets, however, these intermediates may provide substrates for Fenton reaction [2]. The hydroxyl radical, which is a product of Fenton reaction, belongs to the most reactive compounds and may damage virtually all biomolecules. Therefore, despite its low chemical activity, superoxide radical intensively produced in the redox-cycling process may exert its cytotoxic effect in cell [3]. The redox–cycling mechanism, was not observed for resveratrol and its analogues possessing only the resorcinol group (Fig. 1) [2]. Since ROS play a pivotal role in the mechanism of HHRAs cytotoxic activity, it seems obvious that expression of antioxidative enzymes may significantly modulate cytotoxic effect of HHRA exerted on cancer cells. Several studies suggest that MnSOD, the enzyme involved in superoxide radical scavenging, is necessary to maintain mitochondrial integrity in cells exposed to oxidative stress [4] and is crucial for cell survival. We hypothesized therefore, that expression of MnSOD may play a role in oxidative stress induced in cancer cells incubated with stilbenes forming oSQI via dismutation of superoxide radicals produced in redox-cycling processes. In our previous study the importance of MnSOD expression and an impact of oxidative stress on MnSOD expression was evaluated in a breast cancer cell model [5]. Additionally, the effect of superoxide generating resveratrol analogues on tumor suppressor gene p53, which is also activated by reactive oxygen species-generating agents, was assayed. The aim of the present study was to determine the mechanism of action of resveratrol analogues possessing 4 and more hydroxyl groups (Fig. 2) in human cervical cancer cell lines. The experiments were designed to examine the effects of HHRA on cancer cell death, generation of reactive oxygen species and relationship between MnSOD level, p53 status and cytotoxicity of tested compounds. Therefore, the cell lines used in experiments were chosen on the basis of different properties concerning the MnSOD level and p53 status. Our results show that the hydroxylated resveratrol analogues are more effective anticancer agents than resveratrol, which lead to cancer cell death as a result of oxidative stress induction. The C33A cancer cell line (lower level of MnSOD, mutated p53) was more sensitive to HHRAs, compared to the HeLa cell line (a higher level of MnSOD, a wild type of p53), which confirms hypothesis that MnSOD activity affects of cytotoxicity of stilbenes forming oSQI.
 |
Fig. (1). The mechanism of intracellular oxidative stress mediated cytotoxicity of compound possessing pyrogallol, catechol and resorcinol group. |
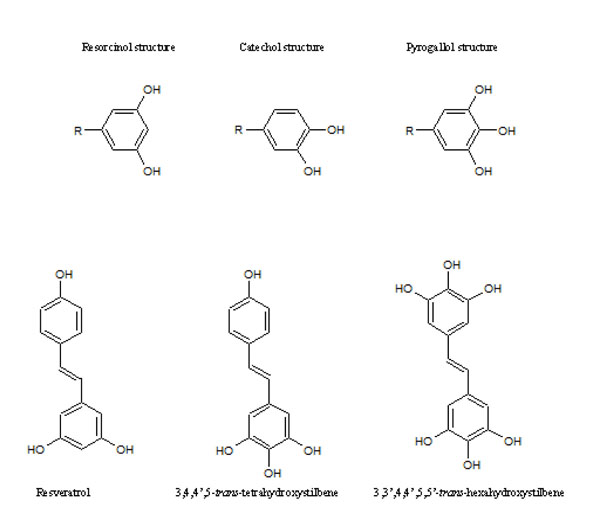 |
Fig. (2). Resveratrol and its analogues tested in experiment. |
MATERIALS AND METHODS
Resveratrol analogues- 3,4,4',5-trans-tetrahydroxystilbene (3,4,4',5-THS) (M8) 3,3',4,4',5,5'-trans-hexahydroxystilbene (3,3',4,4',5,5'-HHS) (M12) were synthesized in the Department of Chemical Technology of Drugs at Poznan University of Medical Sciences using standard chemical methodologies described previously [6]. All the reagents used in experiments (including resveratrol) were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated. All the cell culture consumables were purchased from BD Falcon, (Franklin Lakes, NJ, USA). For all the spectrophotometric and fluorimetric measurements Tecan Infinity 200 microplate reader (Männedorf, Switzerland) was used. Microscopic observations were carried out using Nikon Eclipse TS100 microscope with attached C-SHG fluorescence unit model and DS-SMc digital camera. Pictures were acquired and developed using NIS Elements Basic Research 3.00 software.
Cell Culture
The HeLa and C33A cell lines were obtained from American Type Culture Collection (ATCC). The cells were cultured in DMEM medium without phenol red supplemented with 10%FBS, 1% penicillin/streptomycin and 1% L-glutamine at 37°C, in a humidified atmosphere containing 5% CO2 .
Real-time Quantitative PCR (RQ-PCR) Analysis
Total RNA was isolated according to the method of Chomczynski and Sacchi [7]. The RNA concentration was quantified by measuring the optical density (OD) at 260 nm and their integrity was confirmed by denaturing agarose gel electrophoresis. RNA samples were treated with DNase I and reverse-transcribed into cDNA using oligo-dT primers. The reverse transcription was performed using M-MLV Reverse Transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer instructions.
RQ-PCR was conducted in the Light Cycler real-time PCR detection system Roche Diagnostics GmbH, (Mannheim, Germany) using a LightCycler® 480 Probes Master kit. Target cDNA was quantified using the relative quantification method. The quantity of SOD-2 in each sample was standardized by GAPDH and h MRPL19. For RTq-PCR analysis of SOD-2 mRNA expression, 1 µl of total (20 µl) cDNA solution was added to the mixture of the LightCycler® 480 Probes Master kit (Roche, Mannheim, Germany), primers and probe for MnSOD. In case of a negative control, cDNA was not added.
SDS-PAGE and Western Blot Analysis
The cells were grown in 6 well plates and treated with the RIPA lysis buffer. Next, 30 μg of protein were resuspended in a sample buffer and separated on 10 % Tris-glycine gel using SDS-PAGE. Gel proteins were transferred to nitrocellulose, which was blocked with 5% milk in Tris buffered saline/ Tween. Immunodetection was performed with rabbit polyclonal anti-p53 Ab (sc-101762), anti-MnSOD Ab (sc-30080) followed by incubation with goat anti-rabbit HRP- conjugated Ab (sc-2004). The membranes were also incubated with anti-actin HRP conjugated Ab (sc-1616) to ensure equal protein loading of the lanes. Bands were revealed using SuperSignal West Femto maximum sensitivity substrate Pierce Biotechnology Inc. (Rockford, IL). Densitometric quantification of the band intensity was measured using ImageJ 1.46 software (NIH, USA) and was normalized relatively to the band intensity of the β-actin loading control.
[3H]-thymidine Uptake
For the cell proliferation assay, [3H]-thymidine uptake was used to examine the effect of resveratrol and its analogues on cell growth. Briefly, 1 × 105 per mL cells were treated with various concentrations of tested compounds in 96-well plates. After 48 hours of incubation, 1 μCi [3H]-thymidine (1 μCi/ml; Institute of Radioisotopes, Prague, Czech Republic) was added to the 96 well plates. Subsequently, plates were incubated for additional 12 hours at 37 °C, and the cells were harvested and counted for incorporated radioactivity using a scintillation counter (Wallac Perkin Elmer) [8].
Caspase Activity
The activity of 3, 8, and 9 caspases was performed using a commercially available kit according to the manufacturer’s protocol. C33A and HeLa cells were exposed to resveratrol, M8 and M12 at concentration 50 µM for different time intervals. The hydrolysis of 3, 8 and 9 caspase substrate- - acetyl-Asp-Glu-Val-Asp p-nitroaniline, acetyl-Ile-Glu-Thr-Asp p-nitroaniline, acetyl-Ile-Glu-Thr-Asp p-nitroaniline, respectively, resulted in the release of the p-nitroaniline (pNA) moiety. p-Nitroaniline was detected spectrophotometrically at 405 nm, and the concentration of pNA released from the substrate was calculated from a standard curve prepared with pNA standards. In order to determine assay specificity, additional experiments employing adequate caspase inhibitors were performed [9].
Measurement of Intracellular ROS
The cellular reactive oxygen species generation was determined using 2',7'-dichlorodihydrofluorescein diacetate (H2DCFDA). H2DCFDA is cleaved by intracellular esterases and oxidized by ROS to the 2',7'-dichlorofluorescein (DCF) fluorescent product. HeLa and C33A cells were incubated with 10 μM DCFH-DA in dark for 30 minutes under the cell culture conditions and then cells were exposed to resveratrol, M8 and M12 at concentrations 50 µM for 30 minutes. After incubation the cells were harvested and suspended in HBSS and the fluorescence was measured in Tecan Infinity 200 (Männedorf, Switzerland)microplate reader at excitation wavelength 485 nm and emission wavelength 535 nm [10,11].
Reduced Glutathione (GSH) Level
GSH level in C33A and HeLa was determined using ThioGlo-1 reagent (Calbiochem, San Diego, CA). ThioGlo-1 is a maleimide derivative which reacts with intracellular GSH and gives fluorescent adducts. HeLa and C33A cells were incubated at 37°C for 30 minutes with 10 µM ThioGlo-1, washed with PBS and the level of fluorescence was measured at excitation and emission wavelengths of 380 nm and 510 nm, respectively [12]. The amount of total glutathione was calculated using standard curve with a GSH standard solution.
Mitochondrial Electrochemical Potential Gradient (Δψ)
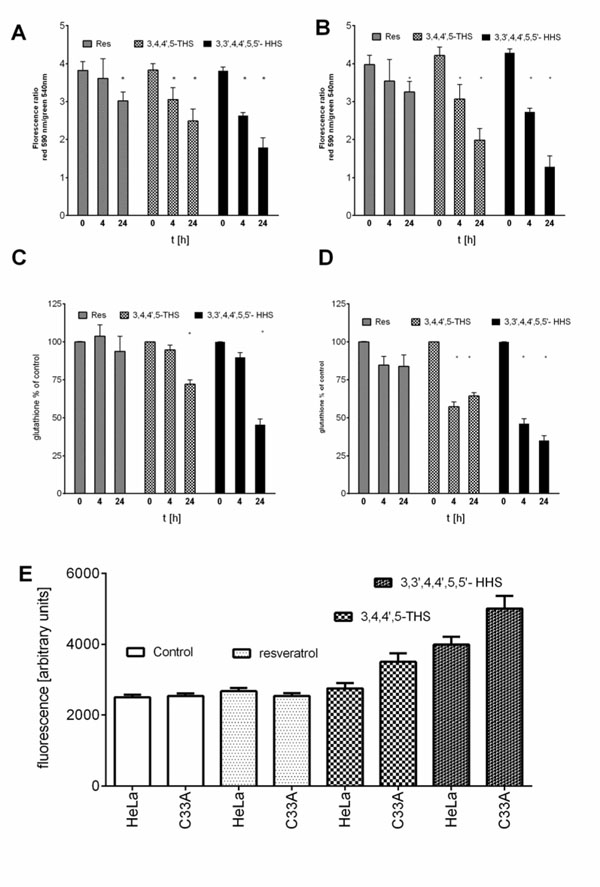
The changes in mitochondrial membrane potential were performed using the mitochondria staining kit (Sigma-Aldrich) according to the manufacturer’s protocol. JC-1 is a lipophilic dye which accumulates in mitochondria in aggregates emitting red fluorescence in healthy cells. During apoptosis or in unhealthy cells, the dye exists in the cytoplasm as a monomeric form emitting green fluorescence [13]. C33A and HeLa cells were treated with tested compounds at concentration 50µM for different time periods (0, 4, 24 h). After incubation the cells were stained with 25 µmol/L of JC-1 for 30 minutes under the cell culture conditions. Fluorescence was measured at wavelengths of 490 nm (excitation)/540 nm (emission) for monomers and 540 nm (excitation)/590 nm (emission) for aggregates form. The changes in the ratio between aggregates and monomers fluorescence intensities at test wavelengths are indicative for the changes in the mitochondrial membrane potential [14].
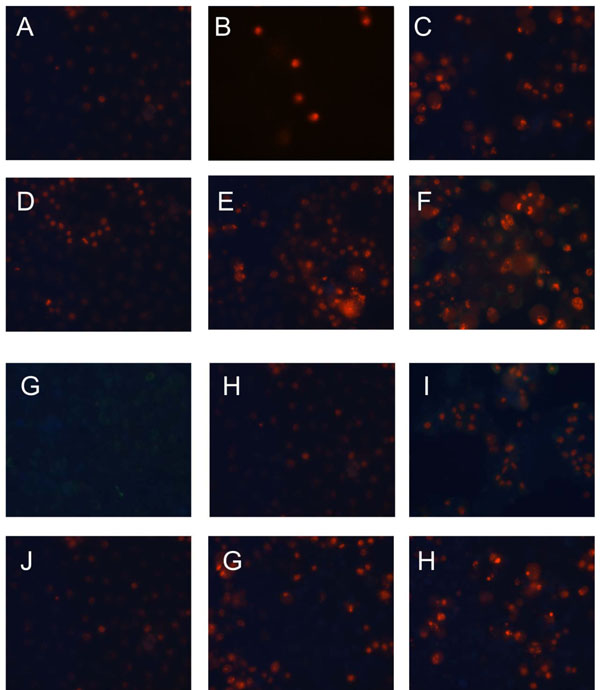
Staining with Annexin-V and Propidium Iodide
Annexin-V-FLUOS Staining Kit was purchased and used according to the manufacturer’s protocol. For this purpose, 8-well chamber slides (BD, San Jose, CA USA) were used. After 24 h of incubation with 50 μM M8, the experimental medium was aspirated and the cells were covered with Annexin-V-FLUOS labeling solution. After incubation for 10–15 minutes at room temperature, cells were analyzed by fluorescence microscopy/ microscope. In the early stages of apoptosis the changes occur on the cell surface. One of these plasma membrane alterations is the translocation of phosphatidylserine from the inner part of the plasma membrane to the outer layer, so that phosphatidylserine becomes exposed on the external surface of the cell. Therefore, Annexin-V stains apoptotic cells. Propidium iodide stains DNA of leaky necrotic cells only, however, late apoptotic cells may be also stained
Statistical Analysis
One-way ANOVA followed by Dunnett’s multiple comparisons test was performed using GraphPad Prism version 6.00 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com
RESULTS AND DISCUSSION
In our experiment expression of MnSOD at the level of mRNA and protein was assayed in HeLa and C33A cancer cell lines using RQ-PCR and Western blot, respectively. We could show that expression of this crucial for antioxidative defence enzyme on both mRNA and protein levels, differs significantly in these two cervix-derived cell lines and is significantly higher in the HeLa cell line (Fig. 3). Next, we assayed an impact of tested compounds on proliferation of HeLa and C33A cells using 3H thymidine incorporation assay (Fig. 4). The inhibition of proliferation of HeLa and C33A cells by resveratrol did not differ significantly for both cell lines and very similar IC50 values were calculated (Table 1). Although, all the tested compounds similarly inhibited proliferation of HeLa cells, both HHRAs, as tested in the experiment, stronger inhibited proliferation of C33A cells than resveratrol. In both cell lines resveratrol induced caspase-3 activity. It should be stressed that activity of caspase-3 induced by resveratrol increased in a monitored time period (0-24hours) and after 24 hours reached about 350% of initial activity in HeLa cells, while in cells incubated with tested HHRAs activity of caspase-3 was significantly lower and declined after a peak which was reached after 6 hours of incubation (Fig. 5). In all the tested cell lines the time dependent loss of the mitochondrial membrane potential was observed; these changes were, however, faster in cells incubated with HHRAs than in cell incubated with resveratrol. Additionally, the loss of mitochondrial membrane potential was observed earlier in C33A cells than in HeLa cells (Fig. 6 A, B). Similar changes pattern was observed in the glutathione level in cytoplasm of cells incubated with resveratrol and its analogues (Fig. 6 C, D). Surprisingly the intracellular ROS level measured using H2DCFDA was not decreased in cells incubated with resveratrol, while in cells incubated with HHRAs the ROS level was significantly higher in both cell lines incubated with M12 and in C33A cells incubated with M8 (Fig. 6 E). The propidium iodide-Annexin V staining in both cell lines incubated with resveratrol showed apoptotic changes after 4 hours (cells stained with Annexin V) while after 12 h late apoptotic and necrotic cells appeared (PI stained cells were observed). In MnSOD overexpressing HeLa cells incubated with HHRAs apoptotic and necrotic changes could be observed after 4 hours and in cells incubated with HHRAs massive necrotic changes were seen after 12 hours. In C33A cells necrotic changes were observed already after 4 hours, similarly to HeLa cells massive necrotic changes in C33A cells incubated with HHRAs could be observed. In the Western blot analyses of p53 level in HeLa and C33A cells no changes in level of this protein in cells incubated with tested compounds were found (data not shown).
Impact of tested compounds on proliferation of HeLa and C33A cells. (incubation 72h, proliferation was measured using 3H-thymidine incorporation assay). The IC50 values are expressed in µM.
| Compound/cell line | HeLa | C33A |
|---|---|---|
| Resveratrol | 17.4±6.5 | 16.7±4.3 |
| 3,4,4’,5-THS | 20.3±3.9 | 7.6±1.6 |
| 3,3’,4,4’,5,5’-THS | 12.2±5.4 | 1.42±5.4 |
 |
Fig. (3). Level of MnSOD mRNA and protein in tested cell lines. |
 |
Fig. (4). Inhibition of HeLa and C33A cells proliferation by resveratrol and its analogues measured using 3Hthymidine incorporation assay. The corresponding IC50 values are presented in Table 1. |
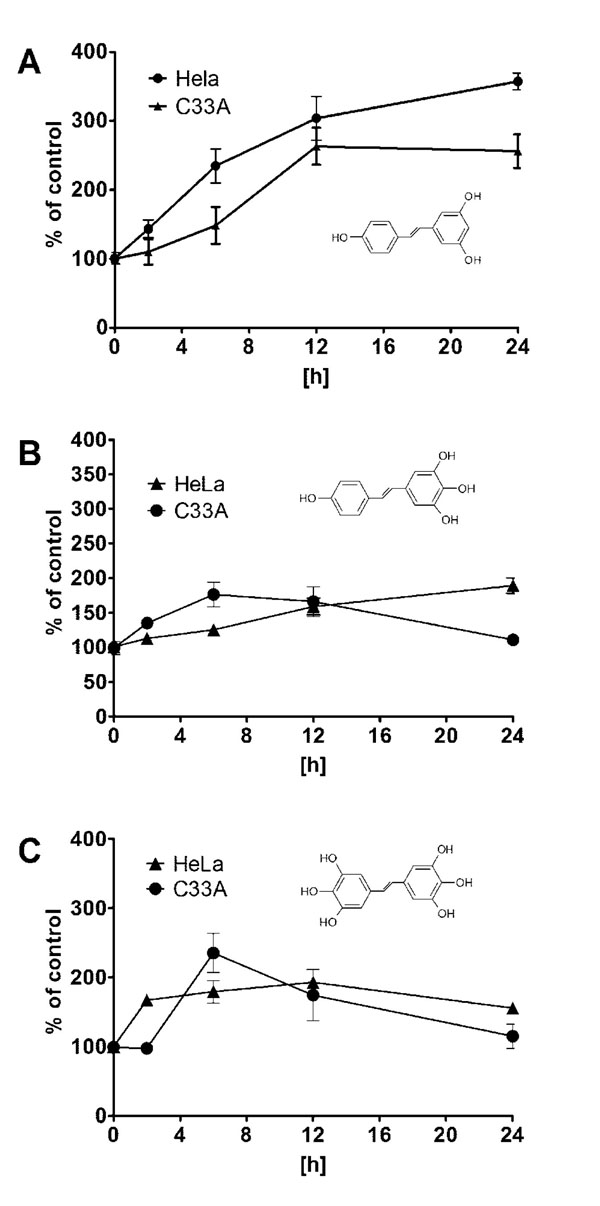 |
Fig. (5). Induction of caspase-3 activity in HeLA and C33A cells incubated with tested compounds (50µM). |
Mitochondria, due to their intensive oxidative metabolism, are believed to be a main source of intercellular ROS. Most of ROS produced by mitochondria originate from the mitochondrial respiratory chain. ROS are considered to be the key players in initiation of neoplasmatic transformation; however, also in transformed cancer cells, they play a very important role in processes involved in both: cell death and cell survival [15, 16]. Due to continuous stimulation by oncogenic signals and mitochondrial malfunctions cancer cells are under continuous oxidative stress [15, 16]. This makes them strictly dependent on antioxidative defense to protect themselves from ROS-mediated damage. The overproduction of ROS is therefore, controlled by antioxidant enzymes and other small-molecule antioxidants [17]. SOD, CAT, GPx belong to the most important antioxidative enzymes. The key role of SOD in antioxidant defense systems was described in several recently published reports dealing with redox physiology of cancer cells [18, 19]. This enzyme is also often proposed as a promising target for anticancer therapy [20, 21]. On the other hand, antioxidant glutathione is considered the most important small molecule [22]. Due to its significant role not only in antioxidative defence but also in conjugation of xenobiotics, its level and cellular turnover are strictly controlled by few enzymatic systems [23, 24]. It was shown in several studies that elevated level of glutathione in many types of tumours may help cancer cells to survive oxidative stress conditions and may also increase resistance to chemo- and radiotherapy [25]. These physiological antioxidative systems may be supported by exogenous antioxidants like e.g. polyphenolic compounds. They are able to prevent damages of cellular macromolecules caused by ROS, as a result of rapid reaction between ROS and biologically important biomolecules like proteins, lipids or nucleic acids. [26,27]. Therefore, due to their antioxidative activity these compounds are considered to be chemopreventive agents able to prevent tumor initiation [28]; polyphenolic compounds, however, are also able to induce apoptosis in cancer cells. Proapototic activity of natural polyphenol like e.g. resveratrol was described in models several cancer cell lines [29,30]. As it was shown before, the higher hydroxylated resveratrol analogues possessing catechol or pyrogallol moiety are the stronger cytotoxic agents than resveratrol or its analogues without such a structure. Experiments employing isolated microsomes suggested that the increased cytotoxicity of ortho-hydroxystilbenes is related to the presence of ortho-semiquinones formed during metabolism or autoxidation. The presence of ROS as a result of involvement of HHRAs in metabolic redox-cycling at cellular level was not confirmed yet because methods of direct ROS detection used recently are still not sensitive enough. The present study provides therefore some indirect proofs that very strong antioxidants HHRAs may exert prooxidative activity in cancer cells. In our experimental model weakly expressing MnSOD cervix cancer C33A cells and HeLa cells highly expressing MnSOD were employed. Significant differences in antiproliferative activity of HHRAs against these cell lines were shown, while antiproliferative activity of resveratrol was very similar in both cell lines. The morphological changes visualized in our experiment in an inverted microscope and Annexin V/PI staining, suggested apoptotic cell death in cells incubated with resveratrol, while in cells incubated with HHRAs apoptotic changes were quickly followed by necrotic cell death. In cells incubated with resveratrol significant increase of caspase-3 was observed, while the activity of caspase-3 was significantly higher in cells incubated with both HHRAs. It has been shown that, oxidative stress may inhibit apoptosis at the level of caspases which require a reducing environment for optimal activity [31]. Apoptosis may be therefore inhibited if antioxidative systems cannot overcome massive ROS generation; in this case, other types of cell death, e.g. necrosis, may occur [32,,33]. Such a scenario could be suggested after analysis of pictures obtained from the cells incubated with HHRAs and stained with Annexin V/PI (Fig. 7).In all the cells incubated with resveratrol or HHRAs, the loss of mitochondrial potential was observed; however, this process was singnificantly faster in cells incubated with HHRAs (Fig. 6 AB). Mitochondria act as stress sensors and the loss of the mitochondrial membrane potential is considered pivotal in cellular apoptosis; however, this cellular event may occur even faster than in apoptosis at the beginning of necrotic cell death [34, 35] which was probaly also observed in our experiment in cells incubated with HHRAs. Although cytotoxic effect of HHRAs may be exerted by ROS generatred in redox-cycling reactions, the ROS level in mitochondria is controled by an efficient antioxidant system composed of mitochondrial superoxide dismutase and the glutathione redox system (glutathione peroxidase and glutathione reductase). This system is used for reduction of superoxide radical, which seems to be crucial problem at this site; however, the second even more important problem may be the product of superoxide dismutation, namely hydrogen peroxide. Since mitochondria are generally unprovided with catalase, the H2O2 detoxification mainly depends on GPx; reduction of H2O2 relies therefore on the GSH level [36, 37] It was shown in several reports that an early disruption of the GSH status, typically within minutes of oxidant exposure, preceded oxidant-induced activation of mitochondrial apoptotic signaling in several cell types [38, 39]. Additionally, it was shown by these authors that the recovery of cellular GSH post-oxidant exposure may be not sufficient to rescue cells from death. This suggests that apoptotic signaling arises when a criticaly low level of GSH is reached and cannot be stopped even if the level of GSH is restored [38-39]. Although, in some of our experiments with other cell lines, e.g. HepG2, the level of GSH decreased shortly after the start of incubation with HHRA and could be restored after several hours, which, however, was not enough to stop apoptotic proceses in thes cells (unpulished data). In both tested cervix cell lines, however, the level of GSH was not restored which may suggest that more severe changes affecting broader spectrum of enzymatic systems were induced in these cells. Taking together, our result may sugget that proliferation of cervix cancer cells, which was measured as decrease of [3H] thymidine uptake is a result of apoptosis/necrosis indcuction by tested, compounds.
Several reports hint that p53 plays an important role in the regulation of metabolism and intracellular redox homeostasis of cells via transcription-dependent and transcription-independent mechanisms. The recent studies suggest that p53 activity may be regulated by mitochondria and cross-talk between mitochondria and p53 is important in normal cellular functions, as well as a breakdown in communication among mitochondria; p53 and the nucleus may have serious consequences in fate of cells [40-42]. It was shown in our previous study employing breast cancer cells that apoptotic process in cells incubated with HHRAs may be mediated via increased expression of p53 [5]. In the current study HeLa cells expressing a wild type of p53 [43] and C33A cells expressing mutated form of p53 were used [44]. In both cell lines incubated with resveratrol and with HHRAs no changes in levels of p53 could be observed.
In conclusion, it may be stressed that mRNA and protein expression of MnSOD, a crucial enzyme protecting cells against superoxide radical generated as byproduct of mitochondrial oxidative metabolism, is significantly different in HeLa and C33A cells (Fig. 2.) As it is was shown before a pyrogallol structure containing stilbene may generate superoxide upon one electron oxidation, therefore HeLa cells highly expressing MnSOD were resistant to 3,4,4’,5-THS and 3,3’,4,4’,5,5’-THS, while C33A cells were much more sensitive. There were no differences in cytotoxic activity of resveratrol in these cell lines (Fig. 3.) Further experiments (Fig. 5.) confirmed induction of oxidative stress in tested cells incubated with 3,4,4’,5-THS and 3,3’,4,4’,5,5’-THS. All the tested stilbens induced caspase-3 activity in HeLa and C33A cells, however, their activity was lower in cells incubated with stilbens containing the pyrogallol structure. It may result from interaction of superoxide radicals with this ROS-sensitive enzyme. Our experiments showed that p53 does not play any role in apoptotic process induced in HeLa and C33A cells by tested stilbens. The results of our study provide indirect proofs, that HHRAs, a very well-known strong antioxidants may act as cytotoxic compounds in cancer cells via generation of ROS and induction of oxidative stress.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
ACKNOWLEDGEMENT
This study was supported by Polish National Science Center by grant number N405 180135 (1801/B/P01/2008/-35).